Abstract
Background: First-line therapy in polycythemia vera (PV) generally consists of low-dose aspirin, phlebotomy, cytoreductive agents, and interferon. Hydroxyurea (HU) is the most frequently used cytoreductive agent, however, about 15-20% of patients (pts) become therapy resistant or intolerant. Resistance to HU is frequently associated with an aggressive disease course by increasing the risk of progression to post-PV myelofibrosis (PPMF). The clinical phenotype at this disease stage is very heterogeneous and often triggers a switch to second-line treatment options like JAK-inhibitor therapy.
Design: A series of more than 500 consecutively collected cases with PV was screened in this observational study and analyzed with regard to inadequately controlled disease and resistance, intolerance or both to first-line cytoreductive agents. Only pts with available bone marrow (BM) trephine biopsies at this disease stage were finally selected. PPMF was defined according to the WHO 2016 criteria and HU resistance was assessed according to the European LeukaemiaNet (ELN). BM trephines were evaluated for grade of BM fibrosis (reticulin/collagen), cellularity, amount of CD34+ progenitors and morphology of megakaryocytes including the occurrence of myelodysplastic features associated with disease progression. Hematological and clinical data were collected at corresponding time points.
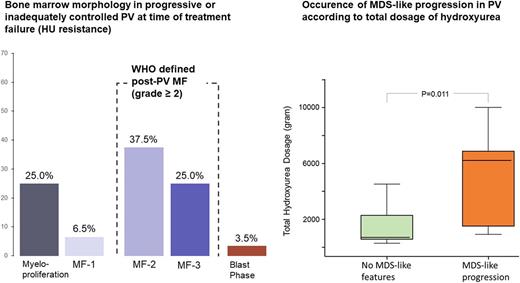
Results: A total of 56 cases (31 males, 25 females, median age 67.5 years) with a representative BM trephine and uncontrolled disease or resistance to first-line cytoreductive agents was analyzed. Median time after diagnosis was 96.5 months (range 4 - 381 months), median treatment time with first-line cytoreductive agents (HU in 87.5%) was 48 months (range 12 - 240 months). Only 62.5% (35/56) of cases presented at this time point with a BM fibrosis grade ≥ 2 and thus fulfilled the WHO criteria for classical PPMF. 14 cases (25.0%) revealed a BM fibrosis grade of 1, whereas an uncontrolled myeloproliferation defined by increased platelet and/or leukocyte counts was found in 7 cases (12.5%). In 2 patients (3.5%) a blast phase was diagnosed at the time of treatment failure which was not associated with progressive fibrosis. In classical PPMF BM cellularity was heterogeneous but generally increased. Cases with grade 1 fibrosis or uncontrolled myeloproliferation had higher hemoglobin levels, lower leukocytes count, and higher platelet counts at time of treatment failure as compared to the PPMF group. On the other site, classical post-PV MF more often was presenting with relevant cytopenia and erythrocyte transfusion dependency. There was no difference in the amount of CD34+ progenitors between classical PPMF and cases presenting with either grade 1 fibrosis or uncontrolled myeloproliferation. MDS-like progression characterized by highly atypical megakaryocytes was observed in 12 pts (21.4%), all pts presented with classical PPMF. For all pts. treated with HU the total cumulative dosage, the average dosage per years, as well as the highest used dosage to control disease was calculated. Pts presenting with MDS-like BM features received a significantly higher total HU dosage (6,230 vs 721 gram, p=0.011) and showed a longer median treatment time with HU (72 vs 48 months), whereas the average annual dosage showed no significant difference.
Conclusions: Progression of inadequately controlled PV represents a biological spectrum and is not restricted by degree of BM fibrosis as in classical WHO defined PPMF, but can also be defined by resistance to cytoreductive agents like HU. In clinical practice it is important to recognize that high dosages of HU and long treatment duration are more often seen in the important subgroup presenting with MDS-like features.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.